+++ latest news +++
Daniel and Marie performed an experimental evolution experiment on Shewanella putrefaciens spreading through soft agar and found something surprising, see here. Great collaboration with Laurence Wilson (York), Rémy Colin (Marburg), and David and Jörn (Bielefeld).
Another great collaboration between the Den and the Bange Lab in Marburg. A long-standing question is how do polarly flagellated gammaproteobacteria spatially organize flagella assembly? Here we finally describe how it works. Now out in Nature Communications.

The Mikrokumpel ('Microbuddy') by Lily has come alive (for example here, or contact us)! An introduction to bacteria (and that they are not all making you sick, but are mostly friends & helpers) for children between 3 and x, with some further information for older readers as well. All income will be donated to early science education and research programs for children.

flagella
cell polarity
phage biology
Our Lab is part of the Institute for
Microbiology and Molecular Biology at the Justus-Liebig-Universität
Gießen, a lively city in the centre of
Germany with Germany's highest student to resident ratio. We are located at the Interdisziplinäres Forschungszentrum (iFZ), a Research Centre for Biosystems, Land Use and Nutrition. Our lab is also part of the SynMikro at Philips University Marburg.
flagella
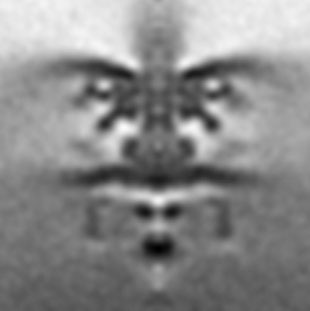
The bacterial flagellar motor is a highly intricate rotating molecular nanomachine. We are interested in the spatiotemporal regulation of flagellar assembly and the various aspects by which flagellar production and functions can be regulated. The cryotomographic image of the Shewanella putrefaciens polar flagella motor was kindly provided by the Beeby Lab.
cell polarity

Numerous fundamental cellular processes in cells (including bacteria) require tight spatiotemporal regulation and coordination. We explore the mechanisms by which bacteria achieve appropriate spatiotemporal organization in particular at the cell pole.
phage biology

Prokaryotic viruses are the most abundant biological entity on earth and affect their hosts in multiple ways ranging from the cellular to the ecological levels. We are interested in the various ways and mechanisms by which bacteria and their phages interact.